Thorne Research Vitamin D K2 Uk
1Sources and Structure
1.1Sources and Intake
Vitamin D is a compound classified as an essential vitamin that derives its name from simply being discovered shortly after Vitamins A, B (prior to the realization that Vitamin 'B' was not a single molecule), and Vitamin C. [3] It was initially found to be a component of Cod Liver Oil, and credited as the 'anti-rachitic' (against rickets) compound to explain how Cod Liver Oil was effective in treating rickets. [4]
Vitamin D is a term used to refer to a group of related molecules, which collectively increase the body's pool of 25-hydroxyvitamin D (circulating form of Vitamin D) and subsequently 1,25-dihydroxyvitamin D (the active hormone).
Food sources of vitamin D3 include:
-
Milk, being the most common source of vitamin D in the USA [5] and has trended downwards in recent decades [6]
-
Cod liver oil at around 2.54-2.78mcg/mL; [7] although labels would be more precise on a product-specific basis as some are lower than this estimate such as 33.5-172.3IU/mL [8] [9]
Dairy appears to be the best food source for vitamin D3. Cod liver oil effectiveness varies, depending on the processor and the method of analysis.
Previously, the RDA was set at 400IU in 1997 (International Units, approximately equal to 10mcg Vitamin D3) as this dose is sufficient to reduce the risk of rickets in children. [10] Even currently, an intake of 400IU (despite keeping the mother in a clinically 'deficient' state according to current definitions) is sufficient to prevent the occurrence of rickets. [11]
This 400IU target intake, as well as the actual overall intake of Vitamin D3, is commonly seen as deficient in adults [12] as 400IU cannot ideally sustain circulating levels between 50-75nmol/L, which is seen as ideal. [13] [14]
The old recommended daily allowance of vitamin D is currently seen as insufficient for adults, despite being sufficient to prevent rickets in offspring. Higher dietary levels are needed.
1.2Synthesis from the Sun
Synthesis of vitamin D can occur after contact with the sun, where bodily stores of 7-dehydrocholesterol (a derivative of cholesterol) converts to cholecalciferol (vitamin D3). [15]
In some scenarios, the rate of vitamin D synthesis is reduced; such as:
-
Latitudes that are further away from the equator tend to reduce synthesis rates due to less exposure to solar radiation. Several studies note that Northern USA (relative to Southern USA) experience less UVB radiation [16], which appears to be related to cancer risk [17]
-
Weather patterns or seasons that reduce solar exposure, such as clouds or darkness [18] [19]
-
A combination of latitude and season, with the Northern Hemisphere (Boston and Edmonton; latitude 42.2-55°N) failing to produce any vitamin D between October and March [20]
-
Areas with higher ozone breakdown (assessed by Dobson units) appear to have higher UVB radiation [21]
-
Darker skin has a slower synthesis rate than lighter skin and Black persons are routinely at a higher risk for vitamin D deficiency when compared to lighter skin tones (Asian, Caucasian, Hispanic) when other factors are controlled for. [22] [23]
Several factors listed above influence the rates of vitamin D synthesis from the sun. The two most relevant factors are latitude, since being closer to the equator results in more vitamin D synthesis, and skin tone, with black people having a higher risk of vitamin D deficiency. An outright failure to produce any UVB-induced previtamin D has been noted above latitude 42.2°N (Boston) from November to February (4 months), which is prolonged to 6 months above latitude 55°N (Edmonton). The range of 18-32°N still produces vitamin D during the winter.
Despite some sunscreen potentially being related to reduced cancer risk from the sun (Melanoma), [24] [25] a topic that is somewhat mixed in results, [26] sunscreens appear to significantly attenuate synthesis of vitamin D by interfering with the topical influences of UVB rays. [27] [28]Chronic (not acute) sunscreen usage has been associated with Vitamin D deficiency. [29]
Sunscreen is able to significantly diminish synthesis of vitamin D, and chronic usage may be associated with vitamin D deficiency, if no oral supplementation exists.
1.3Structure
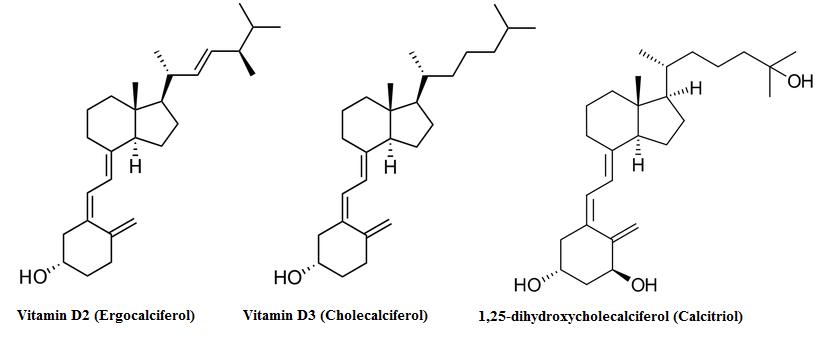
The standard supplement is vitamin D3, otherwise known as cholecalciferol; vitamin D3 tends to be better absorbed than other forms of vitamin D. In the liver, cholecalciferol is turned into 25-hydroxycholecalciferol via the enzyme cholecalciferol 25-hydroxylase and then sent out to the kidneys to get hydroxylated into 1,25-dihydroxycalciferol. 1,25-dihydrocalciferol is also known as Calcitriol, and is the active hormone that is the result of vitamin D3 ingestion.
1.4Bioactivation
Vitamin D is known to be a steroid precursor, which implies that it is currently not bioactive but can become active in the body after metabolism. There are different pathways for oral supplementation and biological synthesis originating in the skin.
When supplementation is not relevant, bodily stores of 7-dehydrocholecalciferol must be converted to cholecalciferol (Vitamin D3). This initial metabolite is present in the skin and the metabolism is initiated by light (spectrum 280-320 UVB [30]) which breaks a part of the molecule known as the B-ring. The metabolite, called pre-vitamin D3, then isomerizes to Vitamin D3 and can then be subject to metabolism in the liver. [30] [31]
The first stage of bioactivation from the molecule cholecalciferol towards the product 25-hydroxycholecalciferol is mediated by a 25-hydroxylase, both CYP2R1 and CYP27A1 being implicated. [15] This process occurs primarily in the liver and due to the next enzyme (CYP27B1) being primarily expressed in the kidneys a large amount of 25-hydroxycholecalciferol is ejected into serum so it can reach this tissue. Upon being subject to CYP27B1 the product is then converted into 1,25-dihydroxycholecalciferol which is considered the active form of Vitamin D as a hormone. [15]
Vitamin D3 is bioactived into its hormone form in either two stages (if starting from a dietary supplement containing Vitamin D3) or in three stages if starting from skin storages, with the skin mediating the first (nonsupplemental) conversion and the later two metabolic steps being handled in the liver and kidneys respectively
1.5Variants of Supplementation
Vitamin D itself is divided into two forms, ergocalciferol (vitamin D2), which is mostly derived from plants, and cholecalciferol (vitamin D3), which is the form produced in mammals and fish and thus is a component of cod liver oil supplementation (alongside vitamin A and fish oil fatty acids). [32] [33] The only difference in these two molecules is a methyl group, as vitamin D3 is 27 carbons in length, while D2 is 28 carbons. [34]
Both vitamin D2 and D3 are seen as prohormone compounds (acting to increase circulating levels of 25-hydroxyvitamin D) [32] although it appears there is controversy over which form is superior in increasing circulating 25-hydroxyvitamin D, with many sources suggesting that vitamin D3 is more effective as the active hormone is 25-hydroxycholecalciferol rather than ergocalciferol (more closely resembling that of D3 than D2 in structure) and that D2 should not be sold as a supplement. [35]
Due to the differences in molecular weight one IU of vitamin D3 is 25ng in weight, while one IU is 25.78ng in weight (the difference being the aforementioned methyl group) meaning that a dose of 400IU for vitamin D3 (10µg) would be 385IU, and this difference was thought to be significant for the prevention of rickets and food fortification.
Vitamin D2 and D3 are two forms of vitamin D supplementation that are capable of increasing circulating levels of the active hormone. Although D3 is more potent than D2 (based on weight), it is (controversially) thought that standardizing the two to an IU value normalizes the difference.
Some studies, such as 11 weeks of supplementation at the winter at 1,000IU (D2 or D3, with a third group given 500IU of each) either as supplementation [36] or orange juice fortification [37] have noted equivalence between the two forms, and elsewhere supplementation of 1,000IU daily in vitamin D deficient persons has noted a difference in circulating hormone levels but no differences in parathyroid hormone. [38]
In a few cases, supplementation of vitamin D2 have increased levels of the molecule 1,25-dihydroxyergocalciferol yet reduced circulating levels of 1,25-dihydroxycholecalciferol. [39]
Other studies using daily dosing of 1,600IU for a year, [34] 4,000IU over 14 days, [40], and for intermittent doses 50,000IU one monthly for a year [34] or a one time dose [41] as well as acute doses of up to 300,000IU D3 [42] have also been noted to be more effective than D2, and according to a meta-analysis the difference in efficacy between D3 and D2 is more notable with bolus supplementation than with daily supplementation. [43]
When comparing D2 against D3 (on an IU basis), there is mixed evidence for both supplements suggesting either bioequivalence (no significant difference) or a superiority of vitamin D3. As there are no studies suggesting that D2 is more efficient, it would be prudent to choose D3 supplementation.
D2 is synthetically produced (for the purpose of supplementation) from irradiation of ergosterol (from mold ergot) whereas D3 is synthesized from 7-dehydrocholesterol. [44]
D2 appears to be less chemically stable than D3 ex vivo (not in an oil base, however) [45] [46] leading to some authors suggesting that it may have a poorer shelf-life. [35]
D2 and D3 are synthesized (for supplementation) by different means, and there appear to be differences in their stability, with D3 being more stable than D2, in powder form.
2Circulating Vitamin D levels
2.1Target Levels
Currently, the generally accepted terms to refer to different possible 'states' of Vitamin D status are: [47]
-
Deficiency (Less than 30nmol/L or 12ng/mL, leading to rickets in children and osteomalacia in adults)
-
Insufficiency (between 30-50nmol/L, the range of 12-20ng/mL)
-
Adequate (between 50-125nmol/L, or 20-50ng/mL)
-
High (above 125nmol/L or 50ng/mL)
(where 2.5 nmol/L is approximately equal to 1 ng/mL, and 1 microgram (mcg or µg) of Vitamin D3 is approximately 40IU [48])
The above are generally accepted guidelines for vitamin D, and will be used as reference for this article. 'Optimal vitamin D levels' is not a legitimate term to refer to one of these four ranges.
A target of 75nmol/L has been considered to as optimal for bone health in older individuals [49] and bone-related conditions such as dental health or reducing the risk of falls and fractures in the elderly. [50] This also appears to be a target for colorectal cancer prevention. [50]
Even in studies that recommend higher oral intakes (5,000IU), the end goal still appears to be around 75-80nmol/L. [12]
Recommended levels of vitamin D are roughly 75nmol/L (30ng/mL) for some conditions.
2.2Deficiency (Predictors)
Vitamin D deficiency appeared to rise from the year 1988 onwards; using 75nmol/L as a cut-off, the percentage of the population below this level increased from 55% to 77% in 2004. [51] It appears to have somewhat stabilized, with 79% below 80nmol/L. [52]
Deficiency rates in the population appear to have increased over the past two decades but they may have stabilized in recent years.
Using other cut-offs, in 2010 29% of the American population was below 50nmol/L (clinical insufficiency) and 3% below 20nmol/L (clinical deficiency). [52] These levels vary by season, and using 50nmol/L as a cut-off again, 11% of people are below this line at the end of summer (testing area of Boston, latitude 42°N), while at the end of winter this number increases to 30%. [53] In a slightly more northern region on the other side of the globe (Britain, latitude 53.1°N) rates of deficiency still increase. When assessing the serum levels of 25, 50, and 75nmol/L the percentage of the population having less than these values increases from 3.2%, 15.4%, and 60.9% at the end of summer to 15.5%, 46.6%, and 87.1% at the end of winter. [54] Estonia (59°N) has the percent of the population scoring below 25nmol/L and 50nmol/L recorded at 8% and 73% at the end of winter, respectively. [55]
Vitamin D deficiency still occurs in locations closer to the equator. One study in Isfahan City, Iran (32°N) had the percent of the population being recorded at below 25,50, and 75nmol/L at 26.9%, 50.8%, and 70.4%, respectively. [56] Cultural and Religious issues may come into play with this study, as the population consisted of both sexes and included women who wear religious clothing in public in this region. Southern Florida (Miami, 25°N) has recorded 38% and 40% of men and women, respectively, at below 50nmol/L. [57]
Despite the above importance on latitude, at least one study has suggested that this may only account for one fifth of the variance seen. [58]
Latitude plays an important role, but deficiency (when defined by serum levels of 25nmol/L or below) and insufficiency (50nmol/L) are prevalent worldwide.
Deficiency is extremely common in medical inpatients, with 22% of patients having serum levels below 20 nmol/L and 57% having levels below 37.5 nmol/L in one study. [59]
Finally, some studies that compare quartiles of Vitamin D levels (dividing the population in to quarters based on the amount of Vitamin D that circulates) find that 50.3% of African Americans are in the lowest quartile of Vitamin D levels (in this study, below 17.3ng/mL [60]) and 7.8% in the highest quartile (32.1%); white people had 9.5% in the lowest quartile and 43.5% in the highest quartile, with Mexican-Americans and all others being split approximately 20%/20% on these quartiles. [60] These results suggest the reduced synthesis rates of vitamin D associated with darker skin hold practical relevance.
2.3Supplementation
Approximately 1000IU daily is needed for 50% of the population to reach 75nmol/L, [50] with 1700IU needed for 95% of the population to reach 75nmol/L. [61] Despite these doses, the human body appears to be able to metabolize more than these levels (up to 3000-5000IU in men [62]) and the body tends to stop solar synthesis (when the UV index is greater than 3) of Vitamin D at a level roughly equivalent to 10,000 IU. [63]
Generally, 2,000IU should be sufficient to meet the needs of most individuals completely, with doses between 2,000-10,000IU not necessarily providing more benefit overall, but not being toxic either.
One meta-analysis of 76 trials [64] has been conducted on serum Vitamin D levels (of people over 50 taking either D2 or D3) with variable daily doses of vitamin D of 5-53.5mcg in most trials with two trials using 124-250mcg daily [65] or 225mcg. [66] When dividing trials by how much vitamin D was supplemented, 10mcg was associated with an average increase of 9ng/mL and an interquartile range (IQR; 25th-75th percentile) of 7.2-14.8ng/mL with double the oral dose (20mcg) being associated with an average serum increase of 12.9ng/mL and an IQR of 9.2-20.4ng/mL. [64] This study calculated (based off meta-analysis) that a predicted increase of 0.78ng/mL (1.95nmol/L) per microgram of Vitamin D3 daily supplement is to not exceed 20mcg (in older adults without calcium supplementation), [64] and similar results have been noted with another review noting that 100IU of Vitamin D3 increases serum Vitamin D by 1-2nmol/L [67] and an increase of 10-25nmol/L with 1,000IU. [68] Despite the first meta-analysis only being conducted in people over 50, this general dose-response over a period of time appears to exist for all age groups. [69] [70] [71] [72]
The main predictors of serum vitamin D levels were form used (vitamin D3 outperforming vitamin D2) and the dose of vitamin D used, which were both statistically significant; coingested calcium supplements and baseline serum vitamin D (lower resulting in a greater increase after supplementation) both trended to increase bioavailability but were not statistically significant. [64] This study could not assess age or gender due to confounds. [64]
In regards to dosing, lower oral doses seem more efficient at increasing serum vitamin D levels, with higher doses still increasing serum levels, but not as much (reduced absorption at higher doses), which underlies variability between individuals. At the lower ranges of oral dosing, vitamin D appears to be linearly increased, with each 100IU increasing serum by approximately 1-2nmol/L and 1,000IU being implicated in the range of 10-25nmol/L (and 2,000IU 20-50nmol/L).
Vitamin D was found to be best absorbed with a low-fat (11 g of fat) meal compared with both a high-fat (35 g of fat) meal and no meal. [73] Further research by the same group found that fat is indeed a central macronutrient for increasing absorption of vitamin D; peak vitamin D plasma levels were found to be 32% higher in subjects consuming a meal with 30 g of fat compared to a fat-free meal of otherwise similar protein content. The composition of the fat (polyunsaturated versus monounsaturated) did not affect absorption. [74]
Vitamin D is best absorbed with a meal, preferrably one with a little fat in it.
20,000IU daily has been associated with toxicity, [63], while daily supplementation of 10,000IU does not appear to induce toxicity, [75]
Sometimes acute boluses are used on a weekly or monthly basis, and toxicity has been associated with a bolus of 300,000IU. [63]
Preloading a large bolus of Vitamin D (in this study, 50,000-100,000IU) prior to a maintenance period does not appear to provide more benefit than simply taking a daily maintaining dose. [76]
Toxicity has been noted at very high daily doses of vitamin D, which are about 10-fold higher than the aforementioned 2,000IU daily dose.
When comparing vitamin D2 against D3, one meta-analysis noted that when they were controlled on a weight basis (micrograms rather than IU) that vitamin D3 was associated with an average serum level increase, which was 4.29ng/mL higher than vitamin D2. [64]
Vitamin D3 appears to be a more reliable form of supplemental vitamin D, relative to D2, for increasing serum levels to an adequate range.
3Lifespan and Extension
Mortality in research tends to refer to death rates from all causes, and its association is established through survey and epidemiological research, since death is infrequent. Causation is almost never established in these instances. Vitality is a general, and mostly colloquial, term used to refer to physical capacity and well being, and is independent of the actual lifespan. Longevity is sometimes seen as a combination of avoiding mortality while promoting vitality.
3.1Mortality
Low vitamin D levels are independently associated with an increase in all-cause mortality in the general population. [60] Smaller samples sizes (derived from NHANES survey data) suggest that this association with mortality is not influenced by gender or by race, and is only dependent on circulating levels of vitamin D, [77] although the higher frequency of low serum vitamin D in blacks (due to less skin synthesis rates) has been shown to increase the overall risk of mortality in an elderly cohort. [78] Another analysis of the NHANES data found that a dose-dependent reduction in all-cause mortality of 6-11% per 10nmol/L increase in circulating vitamin D levels, although this association was borderline insignificant once confounders were taken into account [77] (an increase of 10nmol/L can be obtained by ingestion of approximately 1000IU per day [62]).
When comparing the lower circulating levels of vitamin D against the higher circulating levels in cohort studies, a relationship between risk of death and lower circulating levels are seen; one study noted that those with 50nmol/L (20ng/mL) or less had a relative risk (RR) of all-cause mortality of 1.65. [77] Another study noting that their lowest measured quartile of 17.8ng/mL was independently associated with a 26% greater risk of death relative to the highest quartile (whose serum levels were greater than 32.1ng/mL). [60] Furthermore, in the non-institutionalized elderly, frailty was 1.98-fold higher in the lowest quartile when compared to the highest quartile, and was positively associated with mortality; the lowest quartile had a 2.98 greater relative risk of death compared to the highest quartile. [79] This is important to note as it is suspected that the most benefit against mortality from vitamin D supplementation is a reduction in frailty of the elderly. [79]
A major systematic review consisting and meta-analysis of clinical trials (mainly in the elderly) assessing all forms of vitamin D supplementation has confirmed these observational studies findings of vitamin D's effect on all-cause mortality, finding a relative risk (RR) of all-cause mortality with supplementation of 0.97 (95% confidence interval 0.94-0.99); when specific forms of vitamin D were analyzed, it was found that only vitamin D3 conferred a significant risk reduction (RR of 0.94, 95% confidence interval of 0.91-0.98). [80]
Many observational studies have found an inverse correlation between serum vitamin D levels and all-cause mortality. Clinical trials examining the effect of supplementation on mortality seem to confirm a slight reduction in all-cause mortality, especially in the elderly population. Vitamin D3 supplementation seems to be the most effective form of supplementation with regards to reductions in mortality.
3.2Vitality
In general, supplementation of 1,000 IU vitamin D3 daily (seen as a lower dose estimate) has been estimated to reduce the medical costs of cancer treatment by about $16-$25 billion by exerting a general protective and preventative effect. [81]
3.3Longevity
One study investigating the offspring of nonagenarians (90 or older) with one living sibling (to assume genetic longevity) noted that, when looking at the vitamin D levels of their offspring, that the levels of vitamin D were not elevated beyond that of control; their marital partners. [82] Specifically, the offspring of nonagenarians had 6% less vitamin D and significantly reduced frequency of the CYP2R1 gene that predisposes persons to higher Vitamin D levels. [82]
It is plausible that vitamin D could be a biomarker for something else that is associated with longevity, although no evidence exists to suggest vitamin D can enhance lifespan, just, indirectly of mortality, reduce the risk of premature death.
4Pharmacology
4.1Mechanisms (General)
Vitamin D3 exerts most of its effects either directly via its receptor (the Vitamin D Receptor, known as VDR) acting in the nucleus and promoting protein synthesis, or by a 'non-genomic' action which may still be through the VDR localized not in the nucleus but in cell membrane caveolae. [83]
While classically the VDR was thought to exert its action solely in the nucleus by mediating genomic transcription, it was later shown to translocate from the nucleus towards the cytoplasmic membrane when activated by hormonally active Vitamin D3, suggesting that VDR may play a role in both the genomic and non-genomic actions of vitamin D. [84] Additional evidence for the dual role of the VDR comes from another study in spermatids, which noted that activation of the VDR induced changes in the cell that were abolished by VDR inhibitors yet were not genomic in nature. [85] At the same time, it seems that there are additional membrane-bound non-VDR receptors for vitamin D which may also play a role in the non-genomic actions of vitamin D, such as the 1,25(OH)2D3 Membrane-Associated, Rapid-Response Steroid-binding protein (1,25D3-MARRS, also known as Endoplasmic Reticulum stress Protein 57, or ERp57), which has no sequence similarity to the classical VDR. [86] These two proteins can sometimes work together; for instance, both the VDR and 1,25D3-MARRS have been observed to work in tandem in the case of photoprotection. [87]
Vitamin D exerts its effects secondary to activating its receptors. The classical Vitamin D Receptor can act both genomically and non-genomically, while another receptor known as 1,25D3-MARRS can work non-genomically.
4.2Enzymatic Interactions
Aromatase is an enzyme that is expressed in multiple tissues; one of its primary functions is to produce estrogen locally, which can have a beneficial effect on bone growth and matainance, but can encourage growth of breast cancer tumors. [88] The hormonally active form of Vitamin D3 appears to be a tissue-specific aromatase modulator, increasing its expression in osteoblasts and fibroblasts in bone, [89] as well as adrenocortical [90] and prostate cancer cells (a beneficial effect), [90] [91] and decreasing it in breast cancer cells. [90] Vitamin D3 also appears to induce aromatase activity in placental cells. [92]
A knockout mutation of vitamin D receptors in mice reduced the actions of the aromatase enzyme (CYP19) to varying degrees; activities in the ovaries, testes, and epididymus were reduced by 24%, 58%, and 35% concomitant with reduced gene expression. [93] This may be secondary to disrupting calcium metabolism, since supplementation of calcium to these mice normalized actions of aromatase. [93]
In MCF-7 cells (a breast cancer cell line), 100nM of active Vitamin D3 can reduce aromatase mRNA to 60% of control and almost abolish cultured cell growth in response to incubation of alcohol, which proliferates MCF-7 cells. [94]
Interestingly, a synthetic analogue of Vitamin D3 (known as EB1089) inhibits aromatase via a currently novel inhibitory pathway. [94] This is an analogue that has also shown efficacy in reducing breast cancer in animal models. [95] [96]
Vitamin D appears to be a tissue-selective aromatase modulator, able to increase or decrease aromatase activity dependent on the tissue in question.
In people using pharmaceutical-grade aromatase inhibitors (usually breast cancer therapy), vitamin D levels may be depleted, which predisposes them to musculoskeletal symptoms. [97] although this does not appear to be the most predictive biomarker, with the intentional depletion of estrogen (as treatment for breast cancer) being most predictive and thought to be causative. [98] [99] However, the incidence of joint pain was significantly reduced (odds ratio 0.12, 95% confidence interval 0.03-0.40) in those who achieved serum levels greater than 40 ng/mL with supplementation of 800IU vitamin D daily followed by 16,000IU twice monthly. [100] Higher doses (in this study, 50,000IU weekly of Vitamin D2) appear to be more effective in reducing subjective reports of joint pain. [101]
Vitamin D may attenuate joint pain induced by potent Aromatase Inhibitors (AIs). This may be secondary to potent AIs depleting vitamin D.
The most plausible reason for joint pain with AI usage remains a depletion of estrogens, where depletion of oestrogen is highly associated with induction of athralgia and joint pain.
5Neurology
5.1Mechanisms
Neurons in the brain appear to express the enzyme required to bioactivate vitamin D, [102] with the highest concentrations of this enzyme occuring in the hypothalamus and dopaminergic neurons of the substantia nigra. [103] Most cells express the Vitamin D Receptor (VDR), but it appears to be absent in the nucelar basalis of Meynert and Purkinje cells in the cerebellum, [103] and is expressed in glial cells of the brain. [103]
Calcium metabolism appears to underlie neuronal cell death via excitotoxicity, [104] [105] [106] [107] and hormonally active vitamin D confers a protective effect in vitro at physiologically relevant concentrations up to 100nM but not above. [108] This mechanism of protection appears to be mediated via a downregulation of L-type voltage-sensitive Ca2+ ion channels, [108] an effect which has also been seen in bone cells. [109] [110] These L-type channels have been implicated in excitotoxicity. [111] [112]
One study in rodents have observed such neuroprotective effects in vivo, noting a slower rate of decline in neuronal density in the hippocampus during aging in long-term treatment with vitamin D, indicative of preservation of neuronal cells. [113]
Vitamin D appears to be able to modulate a subset of calcium channels on neurons, and control cell death via excitotoxicity based on in vitro an animal data.
5.2Cognition
In vitamin D-sufficient young adults (30.64 +/- 7.96 ng/mL or 76.6 +/- 19.9 nmol/L), the addition of 5000 IU vitamin D to the diet for one month failed to influence working memory, response inhibition, or cognitive flexibility despite serum levels of vitamin D being increased to an average 39 ng/mL (98 nmol/L). [114] Anxiety and anger ratings were similarily unaffected. [114] However, an 18-week intervention in healthy adults reported that supplementing 4000 IU of vitamin D significantly improved visual memory but not verbal memory, executive function, or working memory. [115] Average vitamin D levels were increased from 25 ng/mL (64 nmol/L) to 52 ng/mL (130 nmol/L). [115]
5.3Depression
A inverse correlation between vitamin D and depression (lower vitamin D status being related to more depressive symptoms) was first reported in 1979 [116] and associations have resurfaced in those at risk for cardiovacular incidents, [117] fibromyalgia, [118] and in women during the winter. [119]
Vitamin D blood levels are inversely correlated with depressive symptoms in some cohorts.
One study noting a correlation between insufficient vitamin D (35-50nmol/L) and depressive symptoms in 54 adolescents also noted an attenuation of symptoms following supplementation of 4000IU for one month and 2000IU for the next two months, where serum vitamin D was increased to 90-91nmol/L (high range of sufficient); a 42% reduction as assessed by WHO-5 rating scale was seen, and improvements seemed universal. [120]
In a randomized, controlled clinical trial high-dose vitamin D supplementation was shown to reduce depressive symptoms in individuals with major depressive disorder (MDD), [121] a condition associated with persistent depressed mood and loss of interest in normally pleasurable activities. [122] In a randomized, double-blinded experimental model, 40 subjects received either a single 50,000 IU vitamin D capsule per week (n=20) or a placebo (n=20) for 8 weeks. Testing with the Beck Depression Inventory (BDI), where a lower score indicates less depressive symptoms, indicated that patients receiving the vitamin D supplement had significantly reduced depressive symptoms at the end of the 8 week trial relative to placebo (-8.0 for vitamin D and -3.3 for placebo, p=0.06). [121]
In contrast, a number of studies have reported that vitamin D fails to alleviate depressive symptoms in various populations. Improvements in depressive symptoms have been noted elsewhere in a small pilot study of women with low (less than 40ng/mL) vitamin D levels and depressive symptoms in the winter months. [119] Conversely, another study noted that with young adults (21.8+/-2.9yrs) with baseline serum levels of 76.6+/-19.9nmol/L (sufficient) given 5,000IU daily for a month, there is no reduction of depression despite an increase of serum vitamin D to 98nmol/L. [114] A failure to reduce depressive symptoms has been noted elsewhere in post-menopausal women given calcitriol supplementation (an active hormonal form of vitamin D). [123]
While some correlational evidences suggests that there may be a link between vitamin D levels and depressive symptoms, the evidence that vitamin D supplementation can help with such symptoms is mixed, and the positive results tend to be in populations with low vitamin D to begin with.
5.4Multiple Sclerosis
Multiple Sclerosis (MS) is a neurological and proinflammatory condition affecting the myelin sheath of neurons, and is the most common neural inflammatory condition in developed nations. [124] [125] A putative connection between multiple sclerosis and vitamin D comes from associations between MS and latitude, which also correlates highly with vitamin D [126] [127] and sun exposure during childhood being inversely related to MS risk in adulthood. [128] [129] No link between maternal vitamin D levels during gestation and risk of MS in offspring has been found, however. [130] Evidence exists suggesting protective effects from sun exposure, [127] [128] [129] with one observational study establishing a protective connection between multiple sclerosis and vitamin D serum levels directly. [130]
Multiple Sclerosis (MS) prevalence is correlated with latitude and sun exposure, both of which are in turn correlated with vitamin D levels.
In animal models of experimental autoimmune encephalomyelitis (a model of MS), Vitamin D can both reduce the occurrence of [131] and slow the progression of the disease. [132] Furthermore, synergism may exist between vitamin D and the standard MS therapy, interferon-beta. [133] The benefits of vitamin D may be related to attenuating demyelination from neurons in vitro. [134]
Vitamin D appears to exert protective effects in an animal model of multiple sclerosis.
5.5Alzheimer's
Alzheimer's Disease (AD) is a neurological condition associated with deficient cholinergic signalling and synaptic function; the mechanisms of Vitamin D appear to hold therapeutic promise for Alzheimer's Disease. [135] Similar to other neurological conditions, Vitamin D in serum appears to be inversely related to AD risk; [136] the risk appears to be a bit lesser than Parkinson's, which have lower serum levels of Vitamin D. [137] [138] Vitamin D receptor polymorphisms have been found in AD, [139] and low serum Vitamin D in older individuals has some predictive validity (assessed by subgroup analysis; small sample). [140]
Although weaker than other correlations, there does appear to be some correlation between Alzheimer's disease and vitamin D.
Vitamin D has been found to stimulate immune cells to catabolize amyloid-β protein aggregates in vitro. [141]
5.6Parkinson's
Vitamin D tends to be inversely associated with risk [142] [143] and its receptor a candidate for Parkinson's therapy [144] as there appears to be an association with one of its polymorphisms and PD risk. [145] Interestingly, persons with Parkinsons appear to have less circulating Vitamin D than do age-matched counterparts with Alzheimer's [137] [138] and appears to exist prior to diagnosis of PD (during early disease pathology) [146] which tends to decline further as the disease progresses. [138] [147]
Low serum vitamin D is correlated with increased risk of Parkinson's Disease (PD) and further associated with the severity of the disease state.
It has been hypothesized that due to the stabilizing effect of Vitamin D on neurons, that deficiency could predispose neurons to toxic stressors. [148] [149] One study that induced Vitamin D deficiency in mice prior to a toxic insult (seen as a method of inducing PD) did not find an exacerbation of damage during deficiency, however. [150] This is in contrast to previous studies in vitro [151] and in animals [152] where excess levels of Vitamin D3 protected neurons from stressors up to 100ng/mL (higher concentrations associated with toxicity). [151]
Mechanistically, vitamin D may protect neurons from stressors, although a deficiency does not appear to inherently increase the risk of neuronal damage on the cells associated with Parkinson's disease.
Currently, there are no clinical trails assessing people with PD and the pathology of PD (cognitive outcomes). Some studies are assessing hip fracture rates, which are covered in the Bone Health section.
5.7Sleep Quality
It has been hypothesized [153] that Vitamin D deficiency is central to a recent 'epidemic' of disturbed sleep patterns [154] [155] that roughly correlates with when the majority of humans began to spend most time indoors. [153]
Some studies in humans suggest improved sleep quality with Vitamin D, but are either done in persons with Chronic Pain being normalized to sufficient levels from deficient [156] or are confounded with other nutrients such as Magnolia Officinalis and Soy Isoflavones. [157] Both studies showed promise, but no controlled trials have been conducted with vitamin D in isolation.
It is plausible that a vitamin D deficiency can hinder sleep quality, and normalizing vitamin D status can normalize sleep function to a degree. There is limited evidence for this relationship at this time.
Vitamin D levels above 85nmol/L (34ng/mL), which are above sufficient, have been anecdonately noted to impair sleep quality, as assessed by REM.
6Cardiovascular Health
6.1Disease Risk
Those with insufficient Vitamin D levels are significantly more likely to develop heart disease than those who do not. [158]
At least one systemic review concludes that 1000IU of Vitamin D daily can reduce the risk of cardiovascular disease based on systemic biomarkers, [159]
However, some individual trials have come up with null results. In one such trial, healthy postmenopausal women given 400IU or 1000IU Vitamin D for a period of 1 year saw no significant benefit to cardiovascular disease risk. [160] In another such trial, people who had vitamin D insufficiency but were otherwise healthy saw no change in several cardiovascular disease markers (blood pressure, LDL-C, HDL-C) when supplemented for 12 weeks with 800 IU vitamin D. [161]
Correlational studies suggest that low vitamin D levels may associated with cardiovascular disease risk. Some, but not all, interventional studies have also found that vitamin D supplementation at moderate to high doses may reduce the risk of cardiovascular disease.
6.2Blood Pressure
Vitamin D was first sought out in relation to blood pressure when it was noted that UV light was able to reduce blood pressure in the general population. [162] [163] In susbequent studies using VDR-receptor knockout mice (mice lacking the Vitamin D receptor, to see what happens in a model of no Vitamin D receptor activity) the mice appear to display increased blood pressure [164] possible secondary to increased serum angiotensin, androsterone, and tissue renin. [165]
Vitamin D appears to suppress Renin via activation of the Vitamin D receptor. Inducers of Renin production tend to work via cAMP as the Renin promoter in the nucleus has many cAMP sensitive response elements, [166] and it was found that Vitamin D can directly suppress renin gene expression via a vitamin D response element that is present in the renin gene. [165]
Vitamin D appears to be a negative regulator of renin expression and reduces activity of the Renin-Angiotension System (RAS). A deficiency of vitamin D lessens the suppression and increases activity of the RAS system, which subsequently increases blood pressure.
A meta-analysis on the topic of Vitamin D and blood pressure [167] investigating eleven trials of persons with hypertension found that noted a reduction in systolic blood pressure that failed to reach statistical significance (95% CI of -8.0 to 0.7) with a small but statistically significant reduction in diastolic blood pressure (95% CI of -5.5 to -0.6) and noted that Vitamin D failed to exert any blood pressure reducing effects in normotensive persons. [167]
One study using 1mcg of active Vitamin D hormone noted that 4 months of treatment was able to reduce diastolic blood pressure in persons with essential hypertension, but only in those with low-renin hypertension. [168]
800IU of Vitamin D3 (with 1,200mg Calcium) has been noted to decrease systolic blood pressure 9.3% in elderly women over 8 weeks, which was to a greater extent than active control (1,200mg Calcium in isolation). [169] However, another study using 800 IU for 12 weeks in healthy vitamin D-insufficient people found no effect on blood pressure. [161]
The reduction in blood pressure associated with vitamin D supplementation in humans appears to be weak in magnitude and possibly dependent on some alteration in metabolism (which would cause hypertension), but it does appear to reduce blood pressure slightly in some people with hypertension.
The blood pressure lowering effect is most likely not strong nor reliable enough to be considered monotherapy to reduce blood pressure, but might be a good complement to other medications.
6.3Cardiac Tissue
In mice lacking the Vitamin D receptor (VDR-/- mice), they appear to have cardiac hypertrophy (up to 22% greater than control mice) as a side-effect [170] which is due to an increase in Angiotension II (AGE II) that has been noted in VDR-/- mice [165] [171] and is known to induce cardiac hypertrophy. [172] [173] Treatment with Captopril, an ACE inhibitor that blocks production of AGE II, reduces cardiac hypertrophy in Vitamin D deficient mice. [170]
Mice lacking the vitamin D receptor appear to have cardiac enlargement due to increased serum angiotension II and increased activity of the RAS system.
6.4Red Blood Cells
Supplementation with 800 IU vitamin D for 12 weeks in people with low vitamin D status but who were otherwise healthy led to small drops in red blood cell count, hemoglobin, and hematocrit when compared to placebo. [161]
6.5Blood Flow
Vitamin D status is associated with arterial stifness and vascular dysfunction in otherwise healthy humans. [174]
Vitamin D levels have been associated with brachial flow-mediated dilation in Type 2 Diabetics. This indicates it plays an important role in heart function, especially in people with disease states. [175]
Vitamin D status might in part help explain the difference in risk of the development of peripheral arterial disease in darker populations (who are more likely to be Vitamin D deficient). [176]
Supplementing 3320IU/d of Vitamin D helped improve several health markers of cardiovascular health during weight loss [177]
6.6Atherosclerosis
It has been noted that endoplasmic reticulum (ER) stress (oxidative stress on a certain organelle in a cell) is pivotall for foam cell production [178] via damaging the macrophage secondary to cholesterol accumulation; [179] [180] macrophages isolated Vitamin D deficient mice appear to be characterized by higher levels of ER stress [164] normalizing this stress with agents known to reduce ER stress normalized the increased foam cell production seem in Vitamin D deficient mice. [164] This suggests that Vitamin D acts to reduce atherosclerosis by reducing oxidative ER stress in macrophages and subsequently preventing foam cell formation. [164]
These effects are mediated by the Vitamin D receptor, [181] and may be related to a shift of Macrophage phenotype from M2 to M1, which appears to be less atherogenic. [182] M2 macrophages (induced by IL-4, IL-10, or immunocomplex) are known to be anti-inflammatory but have a higher potential to accumulate lipids and form atherogenic foam cells [183] [184] while IFN-γ induced M1 cells tend to be proinflammatory and recruits more immune cells but expresses receptors that facilitate macrophage plaque egression and are anti-atherogenic. [185] [186] [164]
Vitamin D appears to act to suppress atherosclerosis by reducing oxidation in macrophages (immune cells) at the level of the endoplasmic reticulum (ER). Stress at the ER causes an accumulation of lipids and cholesterol, which turn into macrophages and subsequently into 'foam cells', which then contribute to plaque. Vitamin D attenuates this process.
7Interactions with Glucose Metabolism
7.1Insulin Sensitivity
Vitamin D levels have been inversely correlated with insulin resistance in non-diabetic adults [187]
Vitamin D levels were inversely associated with serum levels of insulin in adolescents in the United States. People with a serum level of 75nmol/L or more had approximately 24% lower levels of insulin on average than those with lower Vitamin D levels. [188]
Vitamin D levels have an inverse correlation with insulin resistance in both obese and non-obese children. [189]
Vitamin D levels are associated with insulin sensitivity even in non-diabetic adults. [187]
During a glucose tolerance test, subjects who were considered to have insufficient levels of Vitamin D (50nmol/L or less) were more likely to be insulin resistant and have beta cell dysfunction than those who had higher levels of serum Vitamin D. [190]
Supplementation of Vitamin D has been found to improve insulin sensitivity in people who were found to be deficient in Vitamin D, and improve their tolerance to a glucose tolerance test. [191]
In a randomized, controlled clinical trial high-dose vitamin D supplementation was shown to improve markers for glucose homeostasis in individuals with major depressive disorder (MDD). [121] In a randomized, double-blinded experimental model, 40 subjects received either a single 50,000 IU vitamin D capsule per week (n=20) or a placebo (n=20) for 8 weeks. Subjects receiving the vitamin D supplement had significantly reduced serum insulin levels (-3.6 μIU/ml, compared to + 2.9 μIU/ml for placebo, P = 0.06), decreased insulin resistance as estimated by the homeostasis model assessment (HOMA, -1.0 compared to +0.6 for placebo, P= 0.02), and improved beta cell function as estimated by HOMA (-13.9 compared with +10.3 for placebo, P= 0.03). [121]
7.2Diabetes
Decreased serum Vitamin D levels increase risk of the development of Diabetes. [192]
Higher Vitamin D levels prevent the occurence of Type 2 Diabetes. [193]
Low levels of Vitamin D are associated with complications of Type 1 Diabetes. [194]
Vitamin D supplementation improves outcomes of Type 2 Diabetes. [195]
8Fat Mass and Obesity
8.1Associations
It has been hypothesized that Vitamin D insufficiency is a possible contributor to obesity, [196] based on the assumption that serum Vitamin D acts as a sunlight sensor and seasonal and its decline encourages consumption of energy; this consumption of energy to then increase body mass and decrease relative body surface area to confer a thermic advantage in cold environments according to Bergmann's Law. [197] [198] This study attempted to sum up evolutionary theory with the possible mechanism of activating the AgRP/NPY neural circuit while suppressing the POMC/CART circuit of energy intake (although did not provide evidence) with one comment in support of this hypothesis. [199]
Elsewhere, it has been noted that Vitamin D levels are lower in obese persons when compared to controls of similar demographics [200] [201] [202] including pregnant mothers [203] which exists with an increase in serum parathyroid hormone, which Vitamin D normally suppresses. [204] For every 1kg/m2 increase in BMI, it appears that serum Vitamin D is reduced 1.15% (and a 10% increase being related to 4.2% less Vitamin D). [205]
There is a theory that states a deficiency state of vitamin D contributes to the obesity epidemic, but the reasoning is somewhat strained and dependent on caloric overconsumption. An association between lower vitamin D status and obesity has been noted in numerous trials.
8.2Interventions
One study in mice with 10 IU Vitamin D3 per kilogram of feed (relative to control with 1IU/kg) which increased serum vitamin D from around 175 to 425pg/mL noted that fat mass increased independent of overall body weight gain associated with increased PPARγ expression (122% increase), TNF-α secretion (208% increase) and a suppression of UCP2. [206]
In humans, supplementation of 4000IU of Vitamin D3 daily in conjunction with resistance training and a post workout beverage (same in both groups) there was a trend to increase fat mass accrual over the experimental period but this failed to reach significance. [207] Elsewhere, a trial in overweight/obese women given 1,000 IU of Vitamin D daily for 12 weeks resulted in a significant reduction in fat mass (2.7+/-2.1kg lost with Vitamin D, 0.47+/-2.7kg lost in placebo) independent of body weight changes. [208]
There is either no significant effect on fat mass overall or a possible pro-obesogenic effect associated with vitamin D supplementation at high doses. The amount of literature investigating this is admittedly small.
9Skeletal Muscle and Physical Performance
9.1Mechanisms
The Vitamin D receptor (VDR) was thought to be expressed on the nuclear membrane which mediates genomic actions and there appears to be a cytoplasmic membrane receptor which can mediate nongenomic actions [209] such as activation of Protein Kinase C (PKC) [210] which is apparently coupled to a G-protein, [211] Phospholipase D via the same G-protein, [211] [212] and Protein Kinase A2. [213] However, these previous trials appeared to use general (rather than specific) immunostaining (chick monoclonal antibody 9A7 and rabbit polyclonal antibody C-20 both detecting receptors beyond the VDR [214] [215]) to find receptors and a more recent trial using precise VDR immunostaining failed to find any evidence for the expression of this receptor in skeletal muscle. [216] Past studies using autoradiography which confirmed the presence of the VDR in intestinal enterocytes, osteoblasts, parathyroid cells, and distal renal tubules [217] has also failed to detect the VDR in skeletal muscle. [218] [219]
Thus, the detected receptor in the previous studies 'confirming' its actions [211] [212] [213] may be a false positive when investigating another receptor.
There may not be any detectable vitamin D receptors on skeletal muscle tissue, despite a series of studies that suggest this. These appear to be research artifacts caused by inprecise immunostaining techniques.
The nuclear membrane and the actions of Vitamin D appear to be critical for functioning of muscle cells, as otherwise healthy mice who lack this receptor (VDR knockout mice) induces poor swimming ability and induce postural problems which are indicative of poor muscular performance (although these results also implicate the central nervous system and nerve health) [220] [221] as well as 20% smaller muscle diameter. [222]
Despite the lack of vitamin D receptor expression directly on skeletal muscle cells, there appear to be impairments to physical function and reduced skeletal muscle hypertrophy associated with VDR knockout mice.
Skeletal muscle damaged through mechanical scraping showed improved migration when incubated with 10 or 100 nmol 1α,25(OH)2D3 but only the 10 nmol treatment lead to improved myotube number. Creatine kinase activity was also elevated in the 10 nmol treatment condition, above both 100 nmol and vehicle. These data together suggest that vitamin D may play a role in improved muscle recovery after damage, a finding that was tested in vivo in the same study and which is described below. [223]
In vitro evidence suggests that vitamin D may improve muscle recovery after mechanical damage.
9.2Deficiency
A deficiency of Vitamin D is associated with an increase level of fat in skeletal muscle tissue, as assessed by this study in otherwise healthy young women. [224]
In young females, normalizing a Vitamin D deficiency does not appear to confer benefits to hand-grip or pinch-grip strength. [225]
9.3Performance
Supplementation of Vitamin D to correct a deficiency may improve Athletic performance in athletes. A serum Vitamin D level of 50ng/ml (125nmol/L) may be required to do so. [226]
One intervention on sedentary overweight/obese adults given 4000IU of vitamin D daily in conjunction with a resistance training program noted that Vitamin D was assocaited with an increase in power output while placebo was not. [207]
Another intervention on healthy young men found that 4000 IU of vitamin D daily for 6 weeks led to improved muscle recovery after damage induced by eccentric exercise of the quadriceps. The supplemented group was able to generate more torque at a speed of 60 degrees per second at 48 hours and 7 days after the exercise compared to placebo. However, there was no differences between the groups when measuring torque at a higher rate of 180 degrees per second. [223]
9.4Injury and Illness
When assuming an optimal level of 75nmol/L, one study in NFL players noted that up to 64% of athletes had deficient Vitamin D levels, [227] with a correlation existing between players getting injured having less Vitamin D levels. [228]
Vitamin D deficiency appears to be correlated with increased risk of illness and injury among athletes, [229] especially in regards to stress fractures. [230]
10Skeleton and Bone Metabolism
10.1Osteoblasts
Osteoblasts themselves are capable to expressing CYP27B1 and converting inactivate vitamin D (25-hydroxycalciferol) into the active steroid form (1,25-dihydroxycalciferol). [231]
The vitamin D receptor is expressed in osteoblasts [232] where it is involved in controlling their proliferation. [233] In particular, exposure of an osteoblast to vitamin D is known to suppress proliferation of osteoblasts associated with increasing the expression of osteocalcin, bone sialoprotein-1, and RANKL. [234]
Vitamin D acting upon its receptor does promote mineralization of bone tissue. [235]
10.2Fractures
In relatively young and otherwise healthy adults (18-44 years), vitamin D levels in serum are inversely related to fracture risk (military recruits of both genders) with no relation to with BMI nor smoking. [236] [230] When looking at levels of serum intake, there is progressively less risk associated with increasing vitamin D concentrations in the range of 20-50ng/mL [230] ultimately reaching an odds ratio of 0.51 (half the risk at any point in time).
A literature review on the effects of calcium and vitamin D in youth [237] noted that only one prospective study assessed vitamin D, and in this study 800IU vitamin D and 2,000mg calcium was supplemented to female Navy recruits over eight weeks which resulting in a reduction of stress fractures by 21% relative to placebo. [238]
When examining stress fractures in youth, vitamin D is correlated with less risk for fractures. Interventions of vitamin D supplementation appear to further protect individuals from stress fractures.
Trials in elderly indivudals measuring fracture rates have noted a decreased rate in persons with Parkinson's Disease with injections of active vitamin D hormone (reduction of eight fractures over 18 months to one), [1]
10.3Falls in the Elderly
Supplemental Vitamin D, in elderly cohorts, has been noted to reduce the risk of falls by greater than 20% relative to placebo in at least one meta-analysis on the topic; [239] it was suggested that an oral dose of 700-800IU was effective, although one response [240] noted that this dose was not shown to be optimal. Another meta-analysis noted that this risk reduction for falls holds true for persons with low serum Vitamin D, but when including persons with normal serum Vitamin D levels the protective effect dose not appear to be significant. [241]
Supplementation of vitamin D appears to reduce the risk of falls in the elderly, but may only work in people with lower serum vitamin D levels at baseline.
10.4Osteoarthritis
Vitamin D levels in serum appear to predict sensitivity of a joint to heat pain, although they are not related to subjective measures of pain in osteoarthritis. [242]
Serum vitamin D does not appear to correlate with osteoarthritic symptom presence or symptom severity.
In persons with knee osteoarthritis, supplementation of vitamin D3 at 2,000IU daily (dose escalation allowed to assure plasma levels above 36ng/mL) failed to reduce cartilage volume losses and pain symptoms of osteoarthritis (assessed by NSAID usage and WOMAC) relative to placebo. [243]
Supplementation of vitamin D does not appear to significantly reduce joint pain associated with osteoarthritis.
11Inflammation and Immunology
11.1Macrophages
Vitamin D at concentrations above 30ng/mL appears to be associated with less endoplasmic reticulum stress in monocytes, the stress which results in pro-oxidative events that induce adhesion of monocytes to the arterial wall. [244]
11.2Atopic Dermatitis (AD)
Atopic dermatitis (AD) is a chronic inflammatory disease associated with dry, itchy skin and hypersensitivity to allergens. [245] Although the exact causes of the disease are not completely understood, [246] the disorder is associated with improper skin barrier function and over-activity of the immune system, affecting up to 20% of children and 3% of adults. [247] [248]
Given the current lack of understanding of the underlying causes of disease, treatments have been elusive. An emerging body of evidence has implicated low vitamin D levels in a number of cases, however, suggesting that vitamin D deficiency may be a factor. [249]
Atopic Dermatitis, an inflammatory disease associated with dry, itchy skin, has been linked to vitamin D deficiency.
As is the case with many nutritional intervention studies, controlled trials examining the efficacy of vitamin D supplementation for AD have reported mixed results. To examine whether vitamin D supplements may help reduce the symptoms of AD, a systematic review and meta-analysis of the published literature was undertaken by Kim and Bae. [245] Their initial search yielded 266 citations, of which 9 studies met selection criteria for the meta-analysis. The results of the meta-analysis indicated that patients supplementing with vitamin D (dosage range 800-4000 IU, depending on the study) showed a strong trend for reduced severity of AD symptoms. (As assessed in the study by a higher mean difference in the severity of AD symptoms compared to placebo (mean difference = -5.81, 95% CI: -9.03 to -2.59, P= 0.0004)). Although the meta-analysis indicated a strong trend toward reduction in symptoms with vitamin D supplementation, in no case did vitamin D cure the disease. [245] This suggests that although low vitamin D is a factor linked to severity of symptoms, it is not the underlying cause of the disease.
Vitamin D supplementation showed a significant trend toward reduced atopic dermatitis (AD) symptoms across several different trials, suggesting that it may be particularly useful for reducing AD symptoms. Although safe and effective for alleviating AD symptoms, vitamin D is not a cure. More research is needed to uncover, and hopefully cure the underlying cause of disease.
12Interactions with Hormones
12.1Parathyroid Hormone
Serum parathyroid hormone levels are inversely associated with Vitamin D until Vitamin D levels reach between 75 and 100 nmol/L, meaning serum levels below 75 nmol/L might indicate deficient levels of Vitamin D. [250]
12.2Testosterone
In a cross-sectional study assessing correlations between androgens and Vitamin D, it was noted that (n=2299) Vitamin D was positively associated with androgen status (higher testosterone and lower SHBG) even after BMI, smoking, alcohol, beta-blockers and diabetes were controlled for. [251] Additionally, significant correlations were found between androgen status and time of the year, when the sun exposure correlated with higher Vitamin D status, with the peaks (March, August) having 16-18% variation in testosterone levels, but these did not extend to SHBG. [251] Additionally, when investigating serum levels of testosterone and their relationship to falls in the elderly, persons who take Vitamin D/Calcium supplements have significantly less risk additive to testosterone. [252]
In a study on nondiabetics (n=165) where the men were analyzed as a specific subset (n=54), supplementation of 3332IU of Vitamin D daily for a year that was able to normalize serum Vitamin D (increase above 50nmol/L) noted improvements in testosterone (+25.2%), bioactive test (+19%), and free test (+20.2%) in men that were at the low-end of normal for testosterone previously; there was no change in placebo over this time period. [253]
Vitamin D in serum appears to be positively correlated with overall androgen status, with sufficient levels of vitamin D acting to normalize testosterone. There is currently no evidence to suggest supraphysiological levels of vitamin D further enhances testosterone.
12.3Estrogen
Vitamin D appears to regulate estrogen secondary to the aromatase enzyme (converting androgens into estrogens) where deletion of the Vitamin D receptor in mice reduces aromatase activity; calcium supplementation alleviates this suppression, suggesting that Vitamin D regulates aromatase activity via calcium metabolism. [93] This study in receptor deficient mice noted reduced estrogen levels in serum. [93]
12.4Follicle Stimulating Hormone
Follicle-Stimulating Hormone (FSH) is increased in mice lacking the Vitamin D receptor, and this appears to be independent of calcium metabolism. [93]
12.5Luteinizing Hormone
Vitamin D appears to be involved with Luteinizing Hormone (LH), as mice lacking the Vitamin D receptor (abolishing the effects of Vitamin D) appear to have elevated levels of LH; this is not helped with calcium supplementation, suggesting that these effects are independent of calcium metabolism. [93]
13Interactions with Cancer Metabolism
13.1Breast Cancer
When looking at observational studies, Vitamin D in serum appears to be inversely related to breast cancer risk (higher serum levels being associated with lower risk) [254] [255]. Additionally, Vitamin D deficiency appears to be more prevalent in persons with breast cancer (diagnosed) [256] [257] and is similarly correlated with severity of breast cancer. [258] This risk appears more prevalent in black women, where one survey suggested 42% of black women (USA) had serum levels below 15ng/mL (deficiency). [259]
Breast cancer risk is inversely correlated with serum vitamin D levels, which suggests a link between the two.
One large intervention in postmenopausal women (n=36,282) where a smaller cohort had serum parameters of Vitamin D measured (n=1092) following ingestion of 400IU Vitamin D and 1000mcg Calcium daily for 7 years failed to find a significantly reduced risk or breast cancer associated with supplementation. [260] This study reported a 28% increase in serum Vitamin D from 16.9ng/mL to 21.6ng/mL, [260] an increase lower than expected according to a separate meta-analysis where 10mcg Vitamin D3 (400IU) should have increased serum levels to 25.5ng/mL. [64] Another study has noted that 400IU Vitamin D was insufficient to increase serum levels of Vitamin D3 to adequate, [261] suggesting this large study may have used a subactive dose (in addition to compliance issues, if the aforementioned meta-analysis is accurate in its assumptions).
Supplemental ingestion of 400-800IU daily appears to be inadequate to decrease the risk of breast cancer.
Women supplementing with 2000IU/d of Vitamin D may see up to a 50% reduction in the incidence of breast cancer, [254] and another study that noted while 1000IU daily was somewhat effective in improving Vitamin D status that weekly administration of 50,000IU was more effective. [258]
13.2Colon Cancer
According to one systematic review of epidemiological (survey) research (n=30), Vitamin D appears to be inversely correlated with risk of colon and colorectal cancers; [255]
For colorectal cancer outcomes, people with serum levels of 82.5 nmol/L or greater had a 50% lower risk of developing cancer than those with a serum level below 30 nmol/L, and this risk reduction was observed with 2000IU supplemental Vitamin D. [262]
13.3Prostate
Vitamin D appears to be inversely correlated with Prostate Cancer risk according to a systematic review of survey research, looking at 26 studies. [255]
13.4Pancreatic
Doses as low as 600IU/d lower the risk of pancreatic cancer [263]
13.5Ovarian
Vitamin D appears to be inversely related to Ovarian Cancer risk, according to 7 epidemiological studies assessed in this review. [255]
UVB irradiation (which produces Vitamin D) is associated with a decreased risk of developing ovarian cancer in women [264]
13.6Cancer Patients
Vitamin D levels have been inversely associated with BMI in cancer patients. This might be an indicator that nutritional requirements of Vitamin D may be increased for larger individuals. [265]
14Interactions with Lungs
14.1General
In otherwise healthy adults, higher serum Vitamin D appears to be associated with improved lung function as assessed by forced exhalation. [266]
14.2Asthma
Low levels of Vitamin D are associated with increased corticosteroid use in asthmatic children, [267] while supplementation of 1200IU daily is associated with decreased asthmatic attacks in children previously diagnosed with Asthma. [268]
14.3Smoking
A retrospective analysis of survey data between 1984 and 2003 in 626 adult men noted that those without Vitamin D deficiency (serum levels above 20ng/mL) and those who smoked had lower lung function than Vitamin D sufficient smokers, with no relation being found for non-smoking men. [269] From this study, a protective effect on smoke-induced damage was hypothesized.
14.4Respiratory Sickness
Children taking 1200IU of Vitamin D daily were 40% less likely to get the flu during the winter in this study conducted in Japan, [268] while a Mongolian study on children and 300IU daily noted similar benefits. [270]
Post menopausal African women taking 800 IU daily for 3 years were 3x less likely to get the flu than those who didn't. Those taking 800 IU daily for the first 2 years and then 2000 IU daily for the next year were 26x less likely to get the flu. This means supplementing with Vitamin D helps prevent the flu. [271]
One intervention using monthly injections of Vitamin D in otherwise healthy adults (200,000IU for the first two months, 100,000IU for 16 months) failed to find a significant reduction in the frequency of Upper Respiratory Tract Infections in the Vitamin D group among these 322 adults. [272]
Lower Vitamin D levels are associated with an associated with a higher risk of active tuberculosis [273]
14.5Obstructive Sleep Apnea
In correlative research, Vitamin D appears to be correlated with sleep quality. In 190 persons with Obstructive Sleep Apnea (OSA), it was found that patients suffering from OSA had lower vitamin D levels than did the control group and that the lowest levels in the OSA group tended to have the most severe symptoms. [274]
15Interactions with Sexuality
15.1Seminal Parameters
Vitamin D receptors (VDRs) as well as their regulatory enzymes, are expressed in the male reproductive tract; specifically the testes, epididymus and its glandular epithelium, seminal vesicles, and the prostate. [275] [276] This suggests direct actions on sex organs rather than indirect via regulating calcium, which also interacts with sexuality. [277] Vitamin D receptors are also expressed on the spermatid itself, during the late stages of spermatogenesis. [276] [278] Male mice who lack the VDRs suffer from infertility [279] [93] and impaired sperm parameters. [280]
Mechanistically, Vitamin D appears to increase calcium content in spermatids [281] and can act directly on mature sperm cells. [85] Incubation of a sperm cell with hormonally active Vitamin D3 increases calcium influx into the cell secondary to the VDR (due to being abolished by inhibiting the receptor), but not via genomic means and instead via Phospholipase C. [85]
Mechanistically, vitamin D appears to act on the sperm itself (mature spermatid) and improve its motility while enhancing cell survival.
Vitamin D appears to positively correlated with sperm motility, with an assessment of 300 men indicating that those with lower Vitamin D in serum had significantly less seminal motility [281] but that serum levels above 50ng/mL were also associated with less favorable seminal parameters, with an ideal range of 20-50ng/mL. [282] Vitamin D appears to be an independent predictor of seminal parameters in both infertile and fertile men, with more statistical power in infertility. [283]
25-50ng/mL (62.4-124.8nmol/L) appears to be an adequate range to preserve optimal seminal properties in otherwise healthy men, with both lower and higher serum ranges being associated with infertility.
[284]
16Pregnancy and Lactation
16.1Deficiency
Vitamin D deficiency rates appear to be higher in pregnant women than age matched non-pregnant women [285] [286] with deficiency or insufficiency affecting 97% of African-Americans, 81% of Hispanics, and 67% of Caucasians in one trial [287] and another trial in South Carolina (Latitude 32N) noting 48% deficiency rates and 15% sufficiency rates. [288]
This deficiency state has been linked to lower offspring birth weights, which appears to be of most importance in the first trimester, [289] a higher risk of type 1 diabetes developing in the offspring, [290] and higher asthma/rhinitus risk. [291]
In regards to the mother, Vitamin D concentrations below 37.5nmol/L have been associated with an increased need for caesarean section rather than vaginal birth (about 4-fold increased odds). [292] During the first trimester, lower Vitamin D (below 20nmol/L) appears to be associated with greater risk for bacterial vaginosis (57% of women below 20nmol/L; 23% of women above 80nmol/L). [293]
There appears to be lower serum vitamin D in pregnant women, relative to non-pregnant women, with these lower concentrations of Vitamin D being associated with adverse effects for both mother and child. The deficiency appears to be more critical during the first trimester, and thus supplementing vitamin D in response to pregnancy notification (rather than as a daily preventative) may not be prudent and miss time-sensitive periods.
A one time dose of 200,000IU or a daily dose of 800IU during pregnancy has been noted to be insufficient to reach desired serum levels of Vitamin D in pregnant women [294] with dose-dependent increases being noted with daily usage of 2,000-4,000IU (with an author conclusion that the latter is the recommended dosage). [295]
A slightly higher intake of vitamin D may be required to reach sufficiency in pregnant women, relative to nonpregnant women and men, with intakes of up to 4,000IU being advised.
17Interactions with Various Disease States
17.1Lupus Erythematosus
One study lasting 7 months in length using pharmaceutical levels of Vitamin D (100k IU weekly for a month, reduced to once monthly 100k IU for 6 months) noted that, in 20 persons with both Lupus and Vitamin D deficiency, that normalization of serum Vitamin D to 41.5+/-10.1ng/mL was associated with increased naive and regulatory T cell count and reduced memory B cells; possibly beneficial to Systemic Lupus Erythematosus. [296]
17.2Fibromyalgia
One study noted that there was no significant association between Muscle Pain and Vitamin D deficiency when compared to control, but used a control of persons with osterarthritis. [297]
In a cohort of Vitamin D deficient immigrants with complaints of non-specific musculoskeletal pain, once weekly doses of 150k IU Vitamin D3 (19.7mmol/L at baseline, 63.5 nmol/L at 6 weeks and 40nmol/L at 12) reported more reductions of symptoms of muscle pain then placebo (34.9%) and more persons reported an improved abiliy to walk stairs (21.0%), indicative of better muscle function. [298]
Other studies on non-specific musculoskeletal pain note that 50,000IU of Vitamin D2 in 50 persons with diffuse skeletal muscle pain and serum levels belo 20nmol/L failed to significantly improve self-reported ratings of muscle pain (assessed by VAS), although the placebo appeared to elevate their serum Vitamin D levels (thought to have been from sunlight). [297] This same dose was replicated with Vitamin D3 instead of D2, and noted greater improvements than placebo in a Fibromyalgia rating scale; no significant benefit was noted in severely deficient individuals however, and the Firbomyalgia subset was the only one showing improvement (with other subscales not showing significant improvement). [299]
Vitamin D may aid fibromyalgic symptoms (pain and lack of function), but further study is needed.
17.3Sarcopenia
Expression of the Vitamin D Receptor (VDR) in muscle cells decreases with age [300] and Vitamin D deficiency may contribute to an age-related loss of muscle function in elderly persons [301] as well as stand as an independent predictor of muscle strength and mass, with lower serum Vitamin D levels being associated with higher risk of Sarcopenia. [302]
At least one intervention has noted preservation of type II muscle fibers in elderly persons associated with Vitamin D supplementation, [303] and intervention has been associated with improved muscular function in Vitamin D deficiency women. [304]
18Nutrient-Nutrient Interactions
18.1Vitamin K
Vitamin D is potentially synergistic with Vitamin K supplementation as the two share many mechanisms of action in the cardiovascular and bone metabolism systems. [305]
18.2Calcium
People with mean serum levels of 86.5 nmol/L had 65% better absorption of calcium than people with mean serum levels of 50 nmol/L. [306]
19Safety and Toxicity
19.1Kidneys
One Meta-Analysis that examined the link between Vitamin D and mortality (of which a decrease was seen mostly in elderly women) found that there was a higher risk for nephrolithiasis (Kidney Stones) when Vitamin D was paired with Calcium supplementation, with a RR of 1.17 and a CI of 1.02 to 1.34 from a sample size of 74,789. [307] The increased risk of kidney stones and the decreased mortality rates were both only seen with vitamin D3 supplementation. [307]
19.2Squamous dysplasia
High serum vitamin D levels have been associated with esophageal Squamous dysplasia, [308] as one study taking a cross-sectional study of 720 participants in China noted that subjects with Dysplasia had circulating vitamin D levels of 36.5nmol/L while those without dysplasia had 31.5nmol/L and the highest quartile had a relative risk of 1.86 compared to the lowest quartile. [308]
Thorne Research Vitamin D K2 Uk
Source: https://examine.com/supplements/vitamin-d/research/


0 Komentar